HydroGienic® Business Case
Free from traditional constraints
HydroGienic® Business Case
For sectors such as pharmaceutical and biotechnology, high purity water is the lifeblood of manufacturing facilities. Without it, they cannot function, and problematic or inefficient pure water systems will and do, have a massive detrimental impact on production sites.
The disadvantages of conventional water distribution systems (recirculating ring mains) are not fully appreciated, because until now, there has been no effective alternative. This paper outlines a compelling case, both technically and economically.
The proven and innovative HydroGienic® system brings an end to living with the limitations and constraints of conventional pure water piping systems.
Here we outline the capital and revenue benefits of owning and operating a HydroGienic® system, and the significant advantages of this system to pharmaceutical and biotech manufacturers.
Background
We tend to get complacent about water – it is often not appreciated until it is not there, as anyone from a third World country will testify. But even in the developed World, being unable to wash, cook, clean, shower, bathe or flush the WC, soon disrupts everyday life and reminds us of the realities of the importance of water in the domestic situation.
It is the same for many manufacturing industries too, such as pharmaceutical, biotechnology, semi-conductor, photovoltaic etc. who all use large volumes of pure water in their production operations. Further, in many cases of pharmaceutical manufacture for example, pure water is part of the product, e.g. liquids, orals, topicals, opthalmics, inhalations, steriles, injectables etc. In such cases, no water, means no processing, and no product.
Not only is the HydroGienic® water system far superior to existing conventional pure water piping systems in its performance, it can be significantly cheaper and easier to install. It is also capable of delivering significant ‘lifetime cost of ownership’ benefits as are highlighted in this paper, which makes a compelling case.
The HydroGienic® system can be installed alongside existing systems to supply new/additional users, for plant expansions and extensions for example, or as a new build, stand alone system.
HydroGienic® - what is it?
HydroGienic® is a proven and innovative piping system for the distribution of high purity water throughout manufacturing facilities in the pharmaceutical, biotech and related sectors. It is the first of a next generation of systems that will deliver high purity water via a parallel distribution arrangement, providing users with many advantages and benefits over conventional ring mains.
Traditional systems are based upon welded, stainless steel ring mains which connect user points together in series around a recirculating loop. However, these conventional ‘daisy chain’ systems have many limitations and constraints which make them increasingly ineffective and inefficient in today’s manufacturing environment.
Until recent, system owners and users have simply had to tolerate all the disadvantages and inefficiencies of conventional ring main systems as there has been no effective alternative.
HydroGienic® - a summary of how it works
Parallel delivery
The first new and novel feature of HydroGienic® is that it delivers high purity water to each point of use in a system via a parallel piping system. Effectively, this may be thought of as an individual recirculating loop serving each user point via a manifold. Though simple in principle, this change in arrangement is very significant for water system owners and users. It means that each offtake user is individual and separate from the other user points. The result is that -:
- user points can be added, removed or modified on an individual basis – without the need to stop the rest of the system whilst work is being carried out
- the installation project of a new system can begin with a validated distribution system being installed in the plant room with NO users connected. Offtake points can be added singly or in multiples as and when required – a unique and truly modular installation capability.
This is a significant advancement beyond recirculating loops, where all users are impacted during any modifications. Invariably, this means a lengthy system shutdown whenever the conventional loop system is modified or altered. This is extremely costly and inefficient as is described later, but it has been a fact of life for most pharmaceutical and biotech manufacturers before the advent of HydroGienic®.
In comparison, a unique feature of HydroGienic® is that if modifications are being carried out involving a break-in or invasion to the system, all other user points are unaffected and able to remain operable. This translates into potentially huge cost savings, as will be highlighted later.
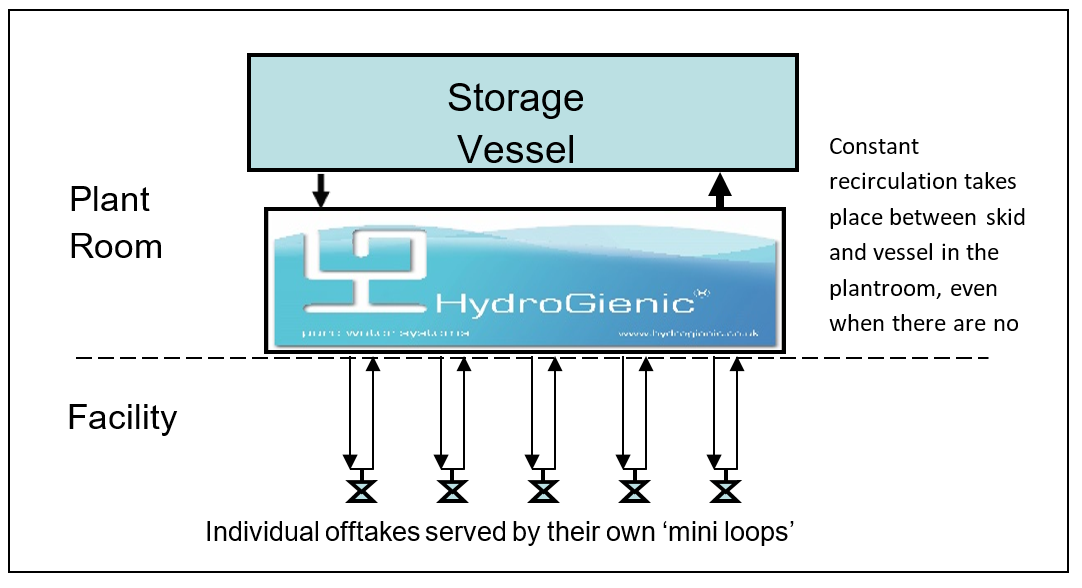
Figure 1 Parallel arrangement of offtakes in the HydroGienic® system
Use of PTFE hoses for delivery
In order to make HydroGienic® cost effective and to make installation simple, a second feature is that small bore PTFE hoses are used for the hygienic pipework delivery. High grade PTFE is already used extensively in pharma and biotech process applications; it has excellent physical and mechanical properties, and can be sanitised using all normal methods such as ozone, other sanitising chemicals, and heat via hot water or steam (eg operating temperature c 230ºC, pressure > 200 barg).
These features lead to it being an ideal medium for high purity water delivery.
HydroGienic® hose installation involves no hot working in production areas, significantly reduced certification and simple materials handling unlike stainless steel welded ring main installations. It is only because of the unique parallel arrangement that small bore PTFE hoses are applicable – they would not be appropriate in large bore, conventional ring main applications.
Typically, the site installation time for a HydroGienic® system would be half that of an equivalent welded stainless steel ring main and installation time from plant room to the user-points is reduced by typically 75%.

Figure 2 HydroGienic® skid at Dechra Pharmaceuticals showing connection of offtake hoses
The business case for HydroGienic®
There is constant economic and commercial pressure on facility managers these days to maximise manufacturing efficiency and output performance, and to minimise costs and overheads. It is therefore sensible to ensure:
- all capital investments and project budgets are cost effective and competitive
- there will be a future return in revenue savings
- there is added value improvements such as to production efficiency and performance and reliability
HydroGienic® can deliver on all three of these things.
There is a tendency for procurement departments to major on the former ie capital savings at the project procurement stage; which is obviously because of having a set budget, and this approach may be fine for commodity items.
However, with more complicated and potentially problematic projects like high purity water systems, there is a strong case for senior managers to look beyond capital cost into added values such as revenue and lifetime benefits, and to consider the impact upon production efficiencies and outputs and risks of getting the wrong, inefficient system.
Capital cost comparison
In terms of capital costs, the HydroGienic® system is often cheaper than the comparable stainless steel ring main system. The HydroGienic® system requires a skid and manifold distribution centre in the plantroom which conventional systems do not, however, once out into the facility, HydroGienic® PTFE hosework will be significantly cheaper to install than welded stainless steel.
It follows logically then, that the bigger the system ie the greater the number of user points and/or distance of pipe runs, the cheaper HydroGienic® will be relative to conventional stainless steel ring mains.
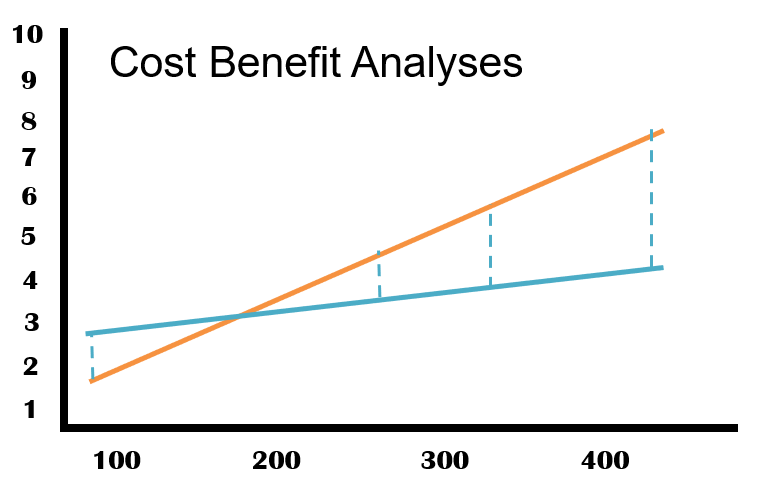
The graph below shows a typical capital cost comparison example between a conventional stainless steel ring main and a HydroGienic® system. Whilst the actual cost comparison will vary based on number of number of user points, the graph clearly shows that the significant cost savings can be achieved where the total loop size exceeds approximately 200 meters based on 2” stainless steel welded pipe.
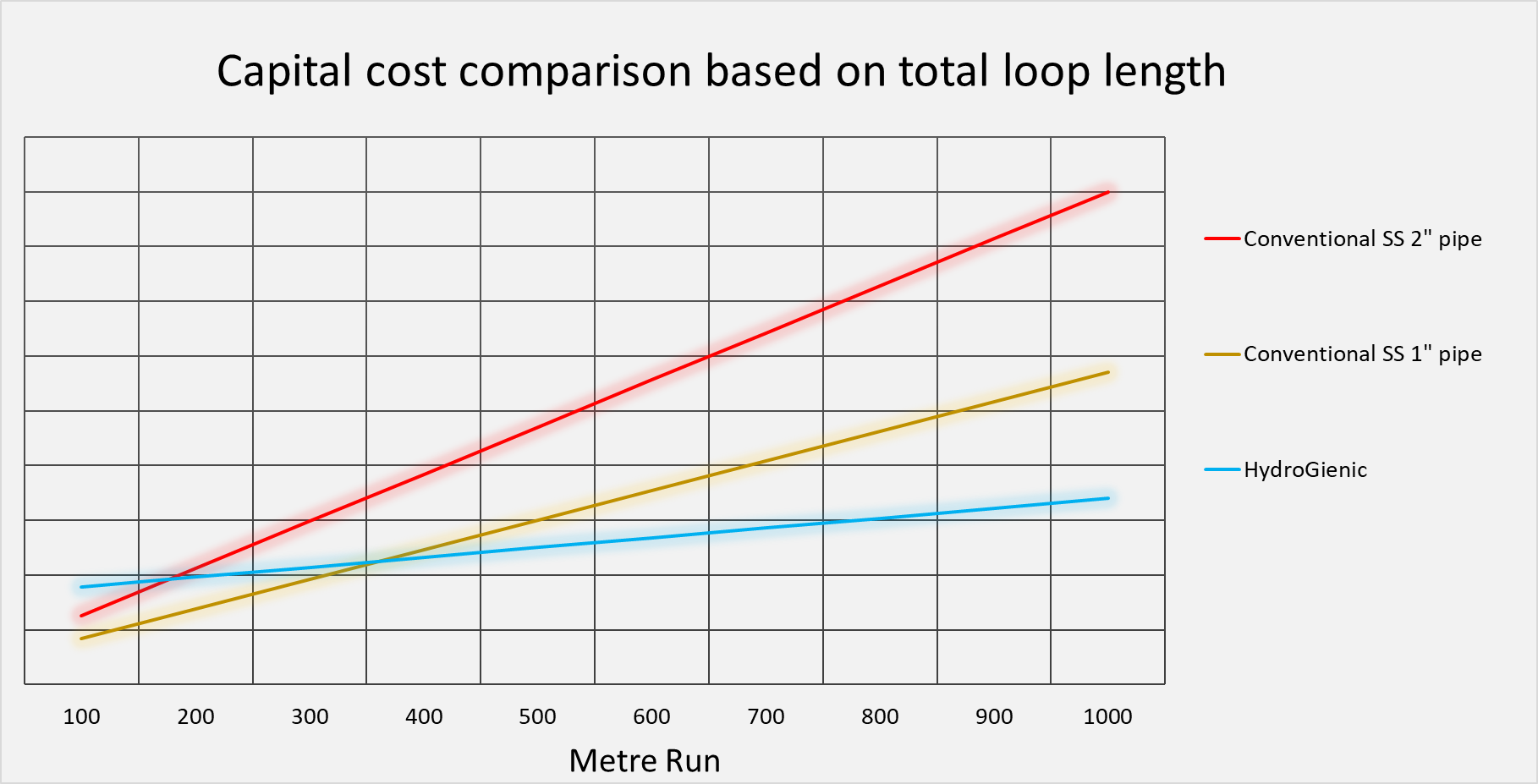
The above representation is based on typical scenarios where good and equal access is available. The more restricted the access becomes, the more challenging the installation of stainless steel becomes and costs can be expected to rise further.
Installation of the HydroGienic® PTFE hoses requires standard cable trays to each user point which can be installed by any approved supplier, with knowledge of the facility, making the installation relatively more straightforward and less time consuming.
Capital cost discussion
The graph below shows a real-life cost comparison between a conventional stainless steel ring main and a HydroGienic® system. For systems with more than 7 or 8 user points, the positive capital cost case for HydroGienic® is clear from this illustration.
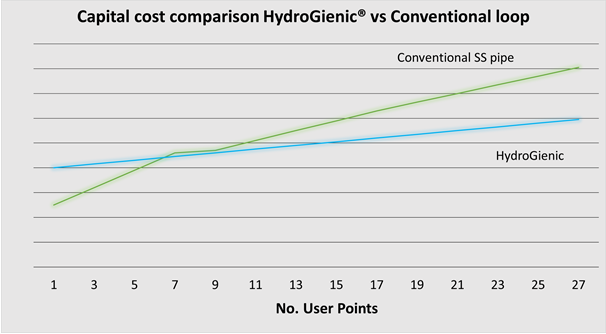
Smaller systems may well be cheaper depending upon user locations, pipe routing etc. All systems will, however small, still benefit from the on-going cost savings and production benefits of the HydroGienic® system.
HydroGienic® can provide capital savings in the order of 25% on systems typical for the industry.
Further to this of course there will be additional performance, revenue and ‘cost of ownership’ benefits which put the capital cost argument somewhat into a different context as will be explained later.
‘Cost of Ownership’ – the real value of HydroGienic®
The real benefit to the HydroGienic® system owner is not actually centred on the capital cost savings, even though there is a strong likelihood that the HydroGienic® system will be cheaper to install than the equivalent conventional ring main as previously illustrated.
The significantly reduced ‘cost of ownership’ is the real prize and the added value of owning a HydroGienic® system, and is the principle reason why it was developed – by users for users.
The parallel piping configuration of HydroGienic® delivers unique adaptability and flexibility of pure water piping as described at the beginning of this article, and these features deliver some truly amazing and ground breaking advantages and benefits to system owners.
These benefits can translate directly into increased production efficiencies and improved production performance over the life time of the system.
Illustration of ‘cost of ownership’ benefits and payback of HydroGienic®
It is highly likely that there will be a need to adapt or modify a pure water piping system during its working lifetime. This may be to add new user points, remove user points, or simply to re-route the piping system to accommodate facility changes and upgrades. All such modifications require a break-in, or invasion, to the system.
It is increasingly likely that such changes are going to become more and more frequent as todays’ manufacturing demands are for more flexible and adaptable facilities. In some sectors, such as the generics market, manufacturers can be modifying and adapting their facilities on quite a regular basis.
There is therefore an increasing industry demand for modular facility construction and adaptability, and conventional stainless steel loops for pure water delivery are simply not designed with that capability in mind.
With a HydroGienic® system, user points can be added and removed without stopping the system, or disrupting the existing users. This can be done without the need for degreasing/passivating, or requalifying the whole system, with minimum fuss and effort.
In a conventional stainless steel ring main, there is quite a rigorous procedure to follow for any invasive modification, which must include -:
- Pre fabrication of new stainless steel pipework sections
- Shut down of the system and draining (NB all user points now disabled)
- Cutting out old pipework as necessary
- Welding in replacement/new sections
- Consideration of degreasing/passivating internal surface of closing welds
- Refilling the system and flush
- Sanitising the system
- Taking samples of water for microbiological testing (change control/PQ acceptance criteria)
- Waiting for usually min 3 days or more for micro results
- Then finally bringing the system back into service
Even after this and production restart, product may be required to be quarantined until requalification data is collected
In addition, this rigorous procedure also carries with it significant risk in those things may not go according to plan or that re-commissioning of the system too early could lead to poor retrospective microbiological data which in turn could even jeopardise product.
There is clearly a very strong argument in favour of HydroGienic® when it comes to system upgrades and modifications. So let’s try and quantify that in financial terms.
The comparative cost of modifying a system and associated production downtime in a number of different types of facility (from generic manufacture, through mid size and large pharma to a large steriles plant) is illustrated below for both a conventional system and a HydroGienic® system.
This simple illustration is based upon the assumption that a typical HydroGienic® system modification might cost around £14K. There would be no need for any additional production disruption costs.
In contrast, a generic company with around 30 to 50 employees might typically lose around £30K per day in lost production when the water system was shut down. For minimum 3 days (assuming the modifications themselves were done at a weekend) that would be a total in lost production of £90K plus say, £15K for the pipework modifications, degrease passivation etc. A total cost for a modification episode of around £105K to a generic manufacturer. The total cost of the modifications is not just limited to the pipework and engineering of the system – there are clearly significant and serious consequences for the impact on the facility whose costs must also be included.
In a mid-sized pharmaceutical plant, employing maybe 50 – 100 employees, lost production could amount to £45K per day. On the same basis, modification to a conventional water system, even with just a minimum of 3 days lost production, could cost the company an estimated total of £150K per episode.
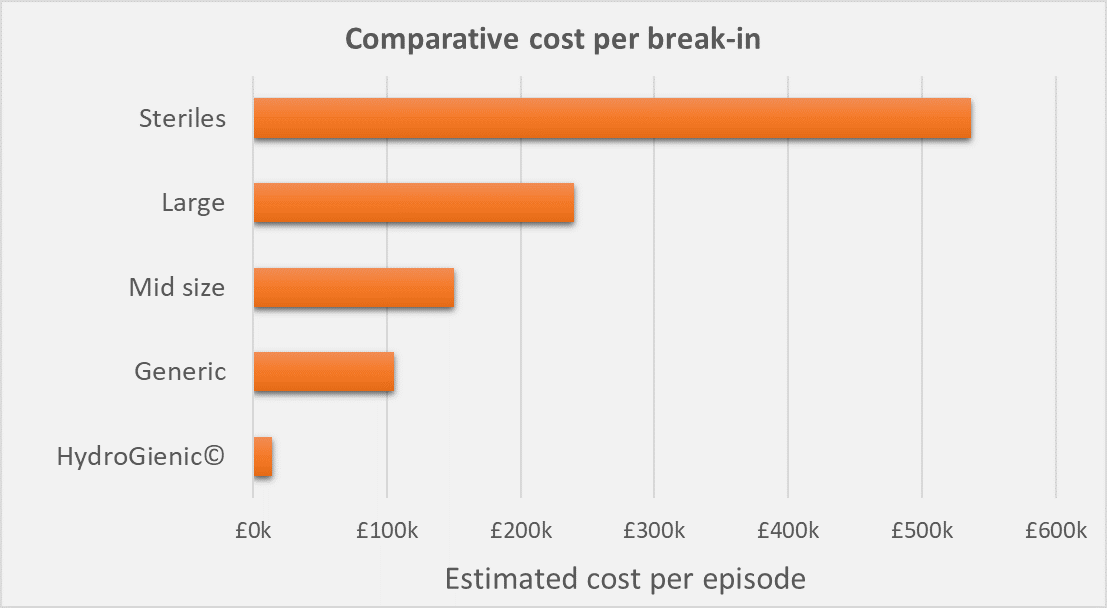
In a larger pharma plant, somewhere around 100 – 200 employees, the lost production costs may be significantly greater. This could be around £75K per day, giving a total cost of around £240K for a 3 day shutdown due to a water system modification.
In higher value facilities, such as large scale sterile plants, loss of production here should the WFI system be shut down, could be typically around £174K per day. That’s a huge cost of £537K per shutdown when the WFI water system is modified.
Of course, these figures are purely illustrative, and a 3 day shutdown is likely to be a minimum (this is optimistic; the true picture could be much worse for conventional systems) but what is clear is that the order of magnitude cost of modifying a conventional stainless steel ring main is very high.
Because of this, water system modifications tend to be ‘put off’ or scheduled in with production maintenance or other activities, or perhaps mitigated by overtime and extra shifts being employed to build up product stocks for the downtime event. These things have a disruptive effect on production, and can lead ultimately to a reduced output and facility performance at the bottom line.
It is not unusual to find that production departments work around the limitations of their pure water systems in this way because of the lack of flexibility of conventional designs.
Lifetime ‘cost of ownership’ vs capital investment
We may quite reasonably assume that a typical pure water system will have a useful working life expectancy of around 20 years. It may also be reasonable to assume that a system modification might take place around every 5 years. (although many systems would be modified much more frequently than that in practice).
Facility owners and production managers are increasingly interested in comparing the lifetime ‘cost of ownership’ with the capital investment of equipment for any project. They are also starting to take a longer term view regarding overall benefits and advantages of investments, in addition to return on capital, and they are evaluating any associated risks before committing to projects.
The data presented in this paper should make that such assessment to be made more easily and with more certainty as far as high purity water systems are concerned.
Discussion
If we accept that the capital cost of a typical Purified Water distribution system may be in the order of say £400K, then not only may a HydroGienic® system be cheaper to install, it also offers potentially massive future savings.
If we look at the lifetime, cumulative ‘cost of ownership’ of this water system, we see an entirely different picture. Let’s assume as stated previously, that the working life expectancy of a typical high purity water system is 20 years.
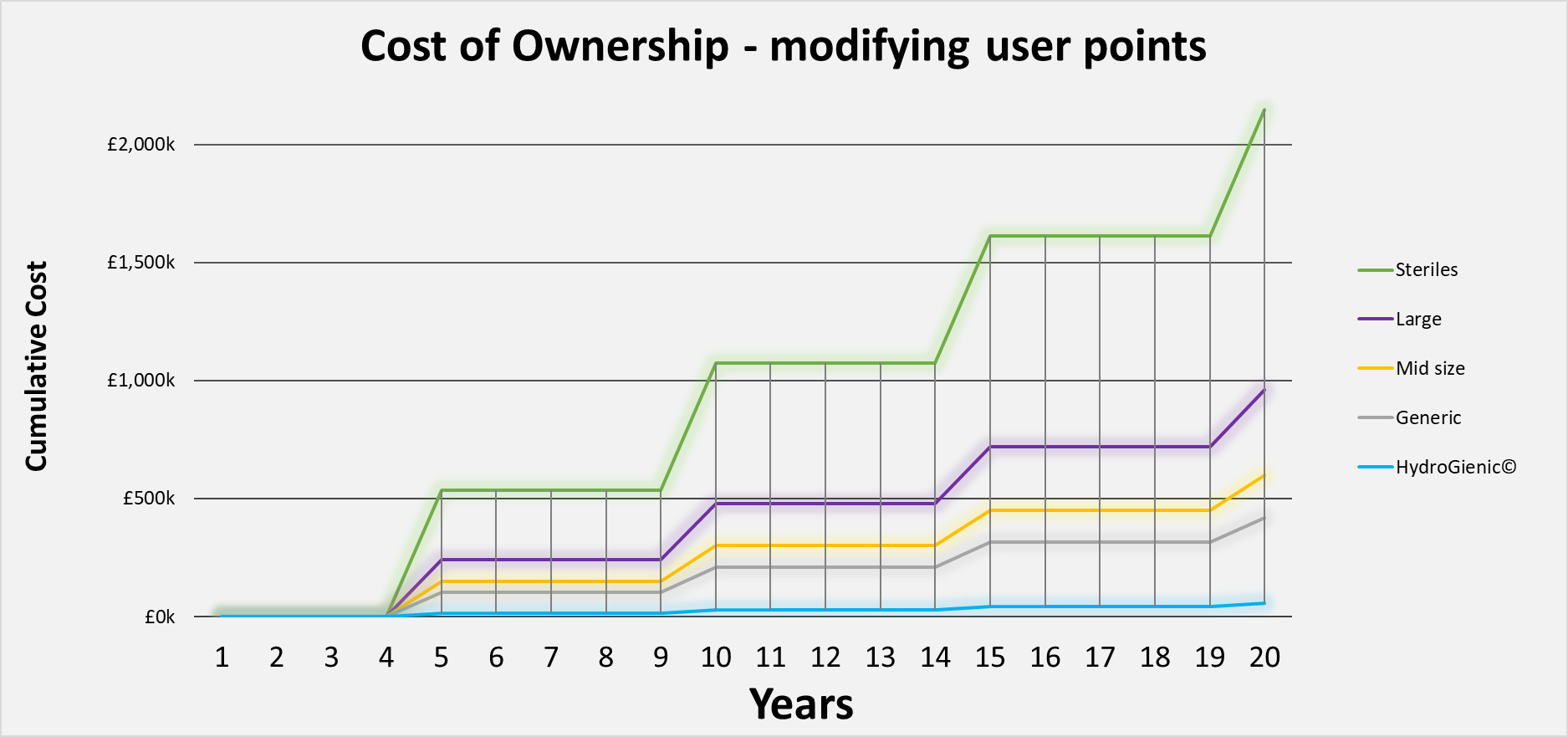
If we introduce a system modification every 5 year, over this period as illustrated in the graph above, (which may be a somewhat modest and reserved assumption), then even in a small generic manufacturing facility this represents a total lifetime saving of £364K. This is from the difference between the HydroGienic® 4-episode total of £56K and the £420K cost of modifying the equivalent conventional system. (That represents 90% of the capital cost of the water system).
In a medium sized plant, the saving would be around £544K – already 36% more than the typical capital cost of the water system itself. In the worst cases – large plants, and high value steriles manufacturing, the ‘cost of ownership’ savings could be £904K and a staggering £2.1m respectively. The latter is FOUR TIMES the capital cost of the water system saved in the lifetime of the system.
Historically, facility managers have probably not looked at their pure water systems in this way. There has been no real need to, because there was no real choice of piping system, and no alternative options.
But the development of new and innovative technology like HydroGienic® should alert senior executives to these issues and considerations. There is an alternative system now, and it is much better, and more cost effective than conventional stainless steel ring mains.
HydroGienic® - technical summary of advantages and benefits
In summary, the unique features of the HydroGienic® parallel configuration and PTFE hose combination provides the following technical and operational advantages and benefits over conventional stainless steel loops -:
- No hot working to install in production areas – minimised facility disruption
- Suitable for all grades of water including PW and WFI, in hot and cold loops
- 75% reduction in installation time from plant room to user-points, 50% reduction in total installation time, reducing overall production down time, project time and cost
- Regulatory compliance; Has passed stringent MHRA and FDA site inspections in working facilities and meets all cGMP requirements and standards
- Delivers 100% diversity i.e. all users can draw water at the same time without impact upon individual user pressures and flowrates
- “Plug and play” addition of user-points with minimal disruption to production
- No need to degrease and passivate the piping system – no harmful chemicals to dispose of
- Allows easy modular facility build- one user point at a time if necessary
- Easy to install along existing conventional pure water systems eg those requiring new or additional users
- Minimises validation effort and cost due to its parallel design and absence of welding inspections
- Significantly reduces the risk of rouge formation (PTFE will not rust)
- Can be sanitised by ozone, elevated temperatures such as hot water and/or steam, and all other common sanitisation chemicals in the same way as conventional loops
Summary
The advent of HydroGienic® and the introduction of a much superior and more elegant design for pure water piping systems should be welcome news for pharmaceutical and biotech manufacturers.
The HydroGienic® system provides owners and system a superior technical solution to pure water delivery, offering consistency, predictable pressure and flowrate performance, meeting all cGMP and regulatory standards and EU/US regulatory expectations, including MHRA and FDA.
In summary, the unique benefits of the HydroGienic® parallel configuration and PTFE hose combination over conventional stainless steel are:
During Install, commissioning and validation;
- 50% reduction in total installation time and 75% reduction in “plant room to user-point” installation time, vastly reducing disruption to the current production area, and reducing overall project time and cost.
- HydroGienic® can provide capital savings in the order of 25% on systems typical for the industry.
- Vastly reduces and simplifies validation effort and cost, as NO hot working is installed in production areas – minimising facility disruption, eradicating the need to degrease and passivate the piping system – no harmful chemicals to dispose of
- Delivers 100% diversity i.e. all users can draw water at the same time without impact upon individual user pressures and flowrates
- Easy to install along existing conventional pure water systems eg those requiring new or additional users without disruption to the existing distribution loop
During its operational life expectancy;
- Lifetime cost of ownership cost reduction of 90% compared to conventional stainless steel loop or an ROI of 400% over 20 years against conventional stainless steel
- “Plug and play” addition of user-points with minimal disruption to production and minimised validation cost due to its parallel design and absence of welding inspections
- Significantly reduces the risk of rouge formation (PTFE will not rust)
For these reasons HydroGienic® should become the preferred choice of pure water delivery system for the pharma and biotech industries in the 21st century. For more information on the HydroGienic® system or to speak to one of our consultants for further information and advice on Water and Steam Systems please browse our knowledge hub.
Author: Trevor Honeyman CChem., MRSC, CSci
Chairman, Honeyman Group Limited
A chartered chemist, Trevor worked for Glaxo, Barnard Castle for 16 years, initially as an analyst then development chemist before being appointed to oversee the management, control and development of all of the site’s pharmaceutical water systems.
The dedicated water section he created, expanded throughout the 1980’s to provide technical support for pharmaceutical water systems throughout the Glaxo Group worldwide, and Trevor developed a wider presence and reputation in pharmaceutical circles for his experience and expertise in this specialised field.
In 1991, Trevor left Glaxo to set up Honeyman Associates, initially an independent consultancy specialising in pharmaceutical water systems and training services. The business expanded with the formation of Honeyman Water in 1997, followed by Honeyman Sterilisation (1999).
The business was streamlined in 2003 by amalgamating the three companies into Honeyman Group Limited to provide the platform from which our baseline expertise in water and sterilisation systems was developed into broader areas of pharmaceutical manufacturing support.
For further information and advice on Water and Steam Systems please browse our knowledge hub.
Download PDF: HydroGienic® Brochure
We understand the sensitive nature of processes and products, to discuss your requirements with one of our experts in a confidential manner, please contact us with your details and a brief introduction, so we can arrange a call back at a time convenient to you.
To Request Further Information Please Contact Us:
Contact UsOur Customers: