Pharmaceutical Water System
GMP Design, Engineering & Process Support Services
Pharmaceutical Water Systems & Processes
GMP Design, Engineering & Process Support Services

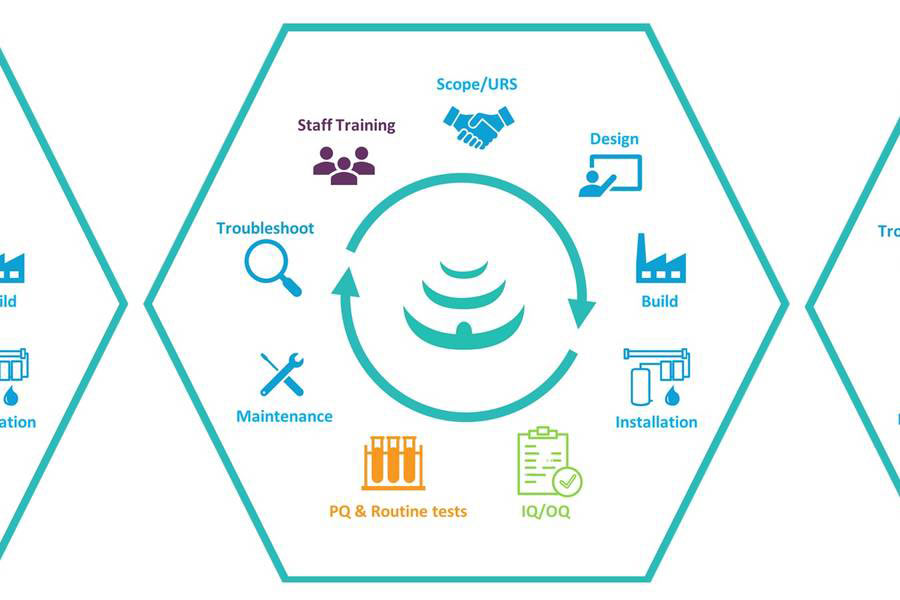
Focusing on the life sciences industry, with extensive knowledge and experience in GMP environments, our dedicated team at Honeyman Water can help you in maintaining a reliable output of compendial purified water, pure steam and WFI provide through a range of different products and services including;
- Pre-treatment and RO-EDI generation systems
- Storage and distribution systems
- System upgrades and modifications
- Routine and call-out maintenance
- Steam Quality Testing to EN285
When quality, compliance, flexibility and efficiency are the essence of your existing or new purified or WFI water system, there is no better place to turn to than Honeyman Water. Experts in high purity water for Pharmaceutical use.
Reducing downtime to improve your bottom line
Contact us to discuss your water system related challenges.
Download PDF: Water Systems Brochure
Download PDF: Site Services Brochure
Pharmaceutical Water System Site Service Maintenance
To Request Further Information Please Contact Us:
Testimonials

Most importantly, Honeyman was meticulous during the system commissioning and qualification phases. The team ensured that every single test was executed and documented to the highest standard.

"Flexibility in expansion with minimal compromise to production, HydroGienic® allows us to do exactly that"

"Our manufacturing business needs to alter schedules quickly and efficiently with minimal compromise to production"

"The new HydroGienic® system flexibility fits with our growth plans. When we need to expand the system, we can increase the number of distribution points with minimal impact on the business"